Assessing stereoelectronic effects in dipolar cycloadditions yielding fused thiazolopyridone rings
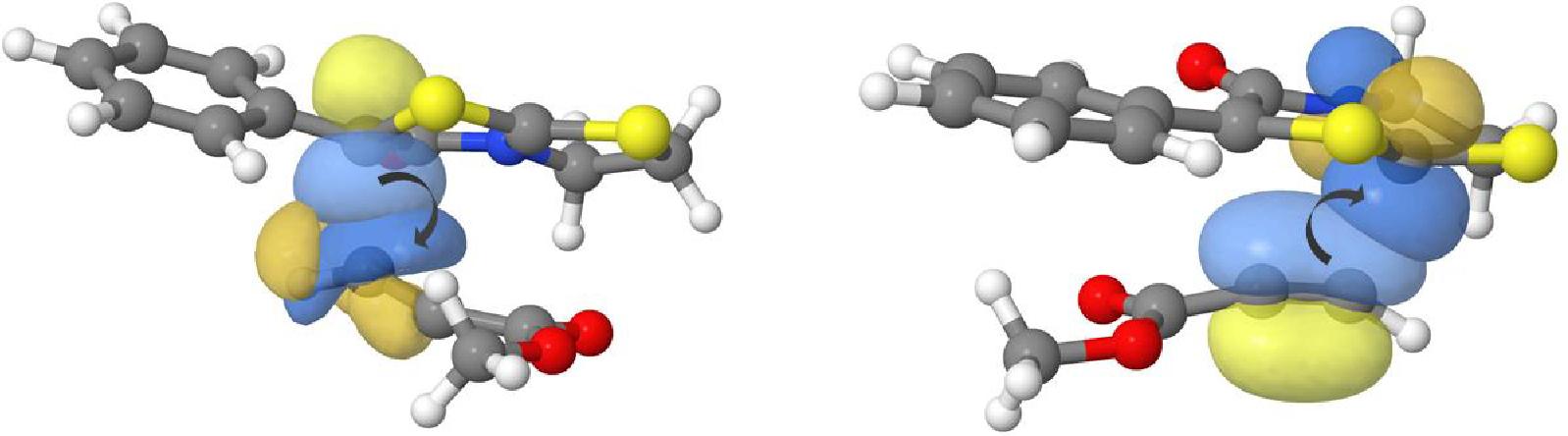
Here is reported a combined experimental and computational study on the cycloadditions of bicyclic 1,3-thiazolium-4-olates, derived from thiazolidin-2-thiones, with asymmetrically-substituted acetylenes. These results provide further mechanistic insights into the above dipolar cycloadditions and enable an unequivocal characterization by NMR spectroscopy of regiochemical patterns as previous derivatives had substituents at both C-2 (in the dipole) and C-6 (in products). Accordingly, new dihydrothiazolopyrid-2-ones have been obtained from a thioisomünchnone lacking substitution at C-2. With the aim of assessing the steric hindrance as well as the facial stereoselection induced by a bulky group on the Si face (relative to C-7a) of the mesoionic heterocycle, a chiral thioisomünchnone has also been obtained along with the resulting optically active thiazolopyridones. A computational study of these particular cycloadditions, largely based on a natural bond orbital (NBO) analysis, allowed us to evaluate the influence of substituents on intermolecular steric repulsions, charge transfers, as well as solvent effects.
- Juan García de la Concepción, Martín Avalos, Reyes Babiano, Pedro Cintas, José L. Jiménez, Mark E. Light, Juan C. Palacios. (2017). Tetrahedron 73 1551-1560.
https://doi.org/10.1016/j.tet.2017.01.064


